Project No. 2123
Primary Supervisor
Dr. Sassan Hafizi – University of Portsmouth
Co-Supervisor(s)
Prof. John Spencer – University of Sussex
Prof. Paul Cox – University of Portsmouth
Summary
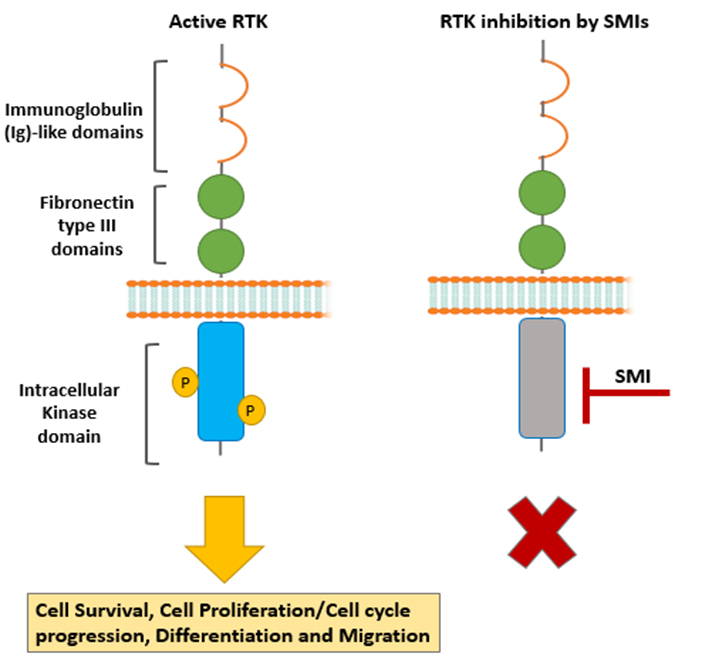
Tyro3 is a receptor tyrosine kinase (RTK) that controls several aspects of cell biology as well as being implicated as a driver of many cancers.
Also, Tyro3’s recognised role in suppression of inflammation/immunity suggests that selective Tyro3 blockade has the potential to inhibit tumorigenesis whilst concomitantly stimulating the anti-tumour immune response. Structural analysis suggests that a small molecule inhibitor of Tyro3 is likely to be more specific than other kinase inhibitors, many of which have off-target effects. However, no clinically viable inhibitors of Tyro3 exist. We have completed a ‘hit identification’ study, combining in silico and in vitro molecular approaches, to reveal three distinct compounds that demonstrate selective inhibition of Tyro3 kinase activity and cancer cell viability. Therefore, this PhD project will be a ‘confirmation of hits’ and ‘lead identification’ study on these novel Tyro3 inhibitors.
Structure-Activity Relationship will be studied for the compounds, using in silico docking analysis to Tyro3 kinase to guide substitutions at identified positions. Compound synthesis will be conducted through organic synthesis schemes, and purity and identity will be confirmed, as well as druggability properties including solubility and stability. The modified compounds will then be investigated in our established assays including Tyro3 kinase inhibition and cancer cell viability. The most promising compounds will be tested against a large array of kinase targets. Furthermore, metabolism of the compounds will be probed in vitro, complemented by in silico analysis.
This ‘hit-to-lead’ study on our existing set of Tyro3 inhibitory compounds should lead to the next stages of drug development, including in vivo efficacy, and further promising results should pave the way towards clinical development as a targeted cancer therapeutic agent. In addition, the agent will be a valuable research tool for further unravelling the role of kinase signalling in maintenance of cellular homeostasis.
