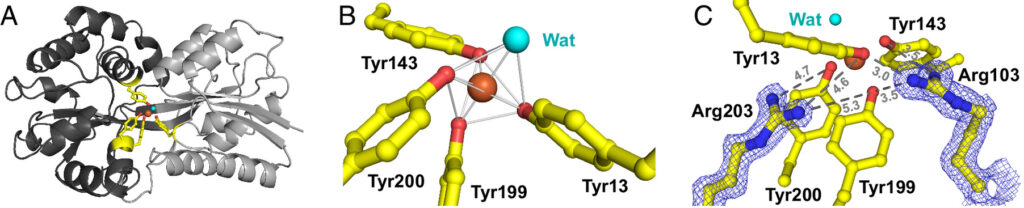
A type of marine bacteria called Prochlorococcus, contributes significantly to global carbon and nitrogen fixation in the oceans. These bacteria are especially adept at surviving in low-nutrient environments. They use a protein called FutA to manage their iron needs, crucial for their survival. The study reveals that FutA can bind to both Fe(III) and Fe(II) oxidation states of iron, which was previously unknown. Through various structural biology techniques at room temperature, researchers reveal a molecular switch on the FutA protein, allowing it to accommodate different forms of iron. This ability is believed to be an adaptation strategy by Prochlorococcus to streamline its genome and cope with iron limitation in its environment.
The paper is a tour de force of structural biology with a variety of techniques and facilities used throughout the study, including conventional rotation crystallography at the National Crystallography Service at the University of Southampton (https://www.ncs.ac.uk), serial synchrotron crystallography (SSX) at the Diamond Light Source/XFEL-Hub (https://www.diamond.ac.uk/Instruments/Mx/XFEL-Hub.html), serial femtosecond crystallography (SFX) at SACLA XFEL (http://xfel.riken.jp/eng/), EPR spectroscopy at the University of East Anglia, in crystallo UV-Vis spectroscopy at the European Synchrotron Radiation Facility (https://www.esrf.fr/home/UsersAndScience/Experiments/MX/About_our_beamlines/icOS.html) and neutron crystallography at the research neutron source Heinz Maier-Leibnitz (https://mlz-garching.de/biodiff).
The research is now published in Proceedings of the National Academy of Sciences (PNAS) (https://doi.org/10.1073/pnas.2308478121).
For more information, please contact Jack Stubbs (jrs1u21@soton.ac.uk).