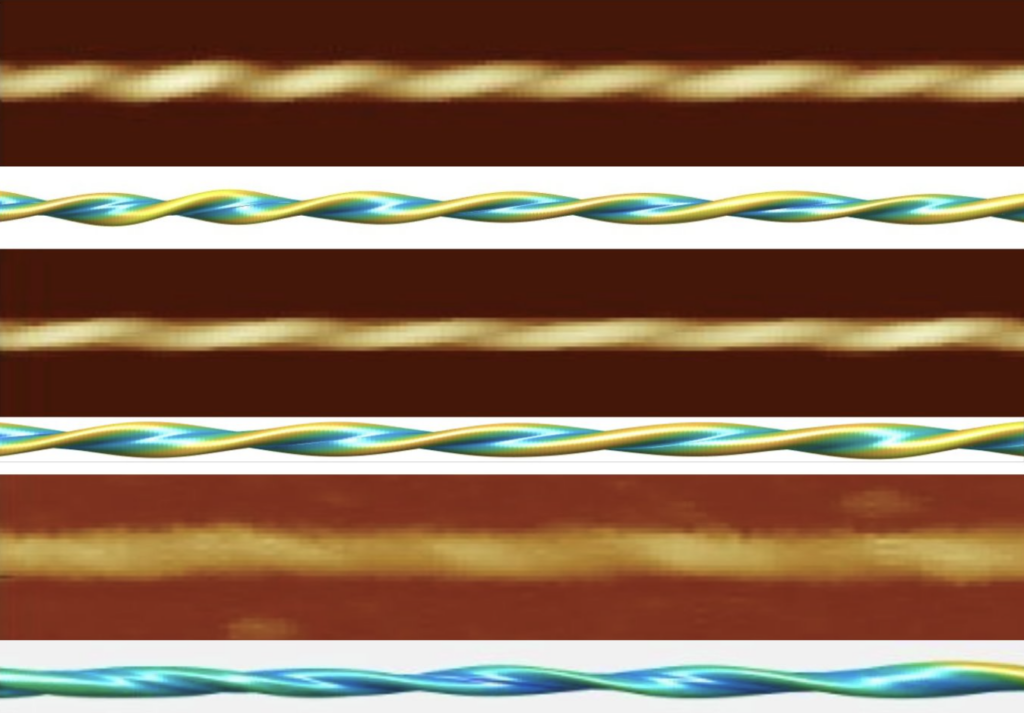
Amyloid structures, recognized for their misfolded protein building blocks that cluster into helical fibrils, are a key factor in neurodegenerative diseases like Alzheimer’s and Parkinson’s. Some amyloid fibrils are associated with biological functions and are candidates to be engineered into novel bio-nanomaterials. Recent breakthroughs in atomic force microscopy (AFM) have significantly impacted our understanding of amyloid structures. The cutting-edge Contact Point Reconstruction AFM (CPR-AFM) method, developed by Dr. Wei-Feng Xue at the University of Kent, unlocks a new dimension of these complex structures, often obscured by conventional ensemble analysis.
CPR-AFM’s innovation lies in its algorithm, which reconstructs the 3D shape of proteins from contact points made by the AFM tip on the protein’s surface. Our review highlights CPR-AFM’s ability to discern subtle structural differences among amyloid fibrils by analysing individual fibrils. Unlike conventional structural analysis methods such as cryo-electron microscopy, which relies on averaging multiple images, AFM provides high signal-to-noise ratio image data,ensuring that individual, potentially rare structures are not masked by averaging, allowing mapping of the structural diversity of amyloid fibrils. This allows potential to develop targeted therapies against disease associated amyloid or engineering strategies for novel nano-material by thoroughly understanding the assembly-structure-function relationships.
I applied CPR-AFM in a synthetic biology initiative that aims to address micro-plastic pollution during my rotation project. This project examines the structures of novel engineered PET-degrading enzyme-linked amyloid filaments derived from the Sup35 yeast protein, enabling us to map the scaffold filaments at different PETase enzyme concentrations. These preliminary findings were presented at the recent AFM and SPM conference, reflecting the promising synthetic biology applications of this research.
Looking ahead, the paper projects an exciting future for AFM technologies, with CPR-AFM working in conjunction with other recent AFM innovations. These advancements are transforming AFM into a versatile, multimodal structural biology tool that allows for an unparalleled analysis of individual biomolecules.
For further reading, the research is published in Chitty, Kuliga and Xue, Biochemical Society Transactions, 2024 (https://doi.org/10.1042/BST20230857).
For more information, please contact Claudia Chitty cc781@kent.ac.uk.
Click here to read full publication