Project No. 2455
STANDARD PROJECT
Primary Supervisor
Dr J. Arjuna Ratnayaka- University of Southampton
Co-Supervisor(s)
Prof Louise Serpell – University of Sussex
Summary
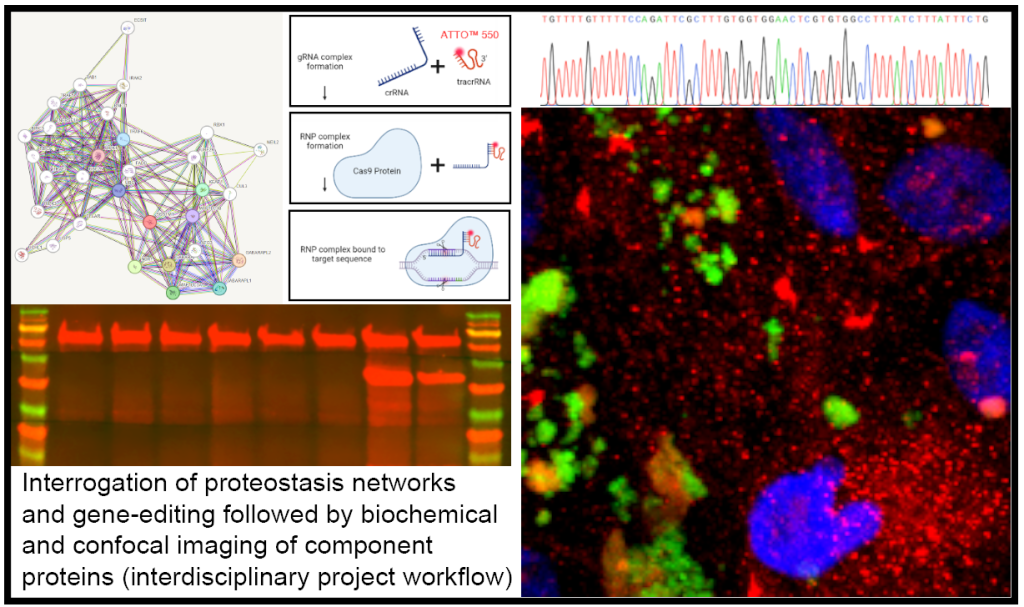
Maintaining proteins that are required for cellular function (or proteostasis) is a feature that becomes impaired with ageing.
Rationale/importance: Maintaining proteins that are required for cellular function (or proteostasis) is a feature that becomes impaired with ageing. Disruption to proteostasis is therefore associated with the accumulation of toxic intracellular macromolecules linked with age-related illnesses; a process that is still incompletely understood(1). Studies to gain fundamental insights into these biological processes typically use a variety of pharmacological compounds/drugs which can lead to artificial conditions. To circumvent these issues, we have developed an elegant in-vitro cellular model to study chronic proteolytic stress in ageing cells.
Methods: Induced pluripotent stem-cell derived retinal pigment epithelial (RPE) and RPE cell-lines that structurally/physiologically recapitulate the native RPE monolayer in the retina will be pulsed with their natural substrate of outer segments. High-resolution imaging and biochemical analyses will generate detailed maps of the proteostasis network including ubiquitination, chaperones as well as the endosome and autophagy-lysosomal pathways. Conditions linked with ageing (increased oxidation etc.) will be overlaid to elucidate how specific pathways affect proteostasis, whilst effects of proteostasis regulatory genes will be tested using CRISPR/Cas-disruption. Assays/reporter constructs will be used to gain insights into dynamic enzymatic responses, ubiquitination levels and trafficking events(2-4). Use of computing/mathematical algorithms, which we have pioneered in our group(5), will provide an unbiased scrutiny of wholescale proteostasis maps. RNAseq studies will reveal cellular responses to disrupted proteostasis at the genetic level.
Impact: This elegant model exploits the unique RPE physiology in the retina, which naturally has the highest proteolytic burden in the body, to provide novel mechanistic insights into impaired proteostasis including unravelling links between different degradative processes in ageing cells.
Candidate attributes: This exciting project is ideal for a highly-motivated candidate with excellent work ethic who is keen to develop skills in cell and molecular biology, biochemistry as well as imaging. An aptitude for computing/coding may also be beneficial.
References:
(1) Impaired Cargo Clearance in the Retinal Pigment Epithelium (RPE) Underlies Irreversible Blinding Diseases. Keeling E, Lotery AJ, Tumbarello DA, Ratnayaka JA. Cells. 2018 Feb 23;7 (2):16. doi: 10.3390/cells7020016.
(2) Impaired lysosomes in the retinal pigment epithelium play a central role in the degeneration of the neuroretina. Miller RD, Ratnayaka JA. Neural Regen Res. 2023 Dec;18(12):2697-2698.
(3) Misfolded amyloid-β-42 impairs the endosomal-lysosomal pathway. Marshall KE, Vadukul DM, Staras K, Serpell LC. Cell Mol Life Sci. 2020 Dec;77(23):5031-5043.
(4) Elevated amyloid beta disrupts the nanoscale organization and function of synaptic vesicle pools in hippocampal neurons. Biasetti L, Rey S, Fowler M, Ratnayaka A, Fennell K, Smith C, Marshall K, Hall C, Vargas-Caballero M, Serpell L, Staras K. Cereb Cortex. 2023 Feb 7;33(4):1263-1276.
(5) An In-Vitro Cell Model of Intracellular Protein Aggregation Provides Insights into RPE Stress Associated with Retinopathy. Keeling E, Culling AJ, Johnston DA, Chatelet DS, Page A, Tumbarello DA, Lotery AJ, Ratnayaka JA. Int J Mol Sci. 2020 Sep 11;21(18):6647.