Project No. 2448
STANDARD PROJECT
Primary Supervisor
Prof Armit Mudher – University of Southampton
Co-Supervisor(s)
Dr Jurgita Zekonyte – University of Portsmouth
Dr Iris Nandhakumar – University of Southampton
Prof Sumeet Mahajan – University of Southampton
Summary
The aim of this proposal is to develop a novel in situ physico-chemical characterisation tool for assessing protein aggregation.
The aim of this proposal is to develop a novel in situ physico-chemical characterisation tool for assessing protein aggregation. This will be achieved by Atomic Force Microscopy (AFM) as well as using surfaceenhanced Raman spectroscopy (SERS) executed through the tip of an AFM probe also called tip-enhanced Raman spectroscopy (TERS). TERS has been shown to achieve single base resolution in single stranded DNA although in dry conditions. Thus TERS can yield morphological, structural (conformation) and chemical level information, all South Coast Biosciences Doctoral Training Partnership (SoCoBio DTP) Email: socobio@soton.ac.uk Website: www.southcoastbiosciencesdtp simultaneously, at the nm-scale that is unparalleled by any other technique. However, TERS imaging under wet conditions is challenging although ~10 nm resolution is achievable with commercially available systems.8 The current state-of-the-art in high resolution protein imaging is Cryo-EM; it has been applied to visualise the folds (~1 nm resolution) involved in Tau fibrils, however, the technique requires huge infrastructure costs, is highly laborious, has very limited throughput and cannot be applied to study native structures in physiological (wet) conditions. Furthermore, applications of TERS in neuroscience and to study aggregation species have not yet been developed. In this proposal we would like to study aggregation using the aggregate prone protein Tau as a model as it forms different aggregate species (especially oligomers and fibrils) in a physiological environment. Application of TERS will thus allow unprecedented insight into chemical composition and conformation at the single oligomer and single fibril level. We will also develop the TERS technique to achieve ~1 nm resolution so that we can obtain conformational information at the level of residues (amino acids) in Tau protein and its aggregates, which will be groundbreaking.
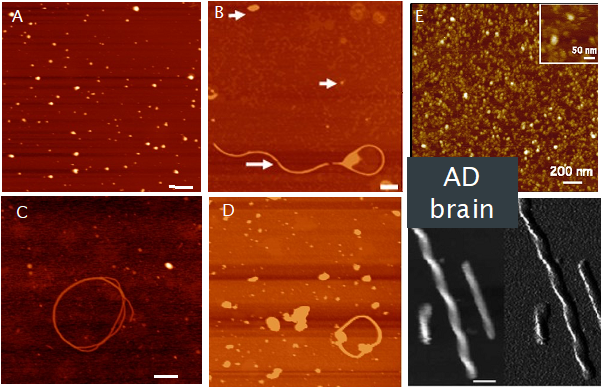
Human tau aggregates in adult fly brain
(A) Oligomers of tau evident following expression of hTau in adult photoreceptor neurons at 0wk; B) Oligomers (arrowheads) and longer filaments seen following pan-neural expression of hTau after 5wks; C) Co-expression of the tau kinase glycogen synthase kinase (GSK) accelerates filament formation in photoreceptor neurons (0wk); D) mCherry tag does not alter tau aggregation into oligomers or filaments – pan-neural expression 5wk. E) Tau extracted from AD brains forms oligomers and fibrils that are morphologically similar to those formed in fly brain. Scale bar 200nm
